Ingredients can come in many different forms. Look at creatine for example; it is available as creatine monohydrate, creatine HCL, creatine ethyl ester, and many other forms. Each form of creatine has a supposed, often unsubstantiated, benefit over the other forms. Potential benefits could include better bioavailability/absorption, solubility in water, and taste. Companies may use an inferior form of an ingredient, knowingly or unknowingly, to market the supposed benefit or to improve the consumer sensory experience (i.e. make a product mix better in water). In the case of creatine, creatine monohydrate hands down has the most scientific substantiation and is the superior form of creatine, but companies still use other forms of creatine for marketing reasons.
A current trend in supplement formulation is to use N-acetyl tyrosine (NAT) instead of L-tyrosine because NAT is more soluble and mixes much better in water than L-tyrosine. NAT’s increased solubility should in theory make it more bioavailability than L-tyrosine. But is this true? Is NAT superior to L-Tyrosine? Let’s dive into scientific literature to find out!
Tyrosine vs. N-Acetyl Tyrosine
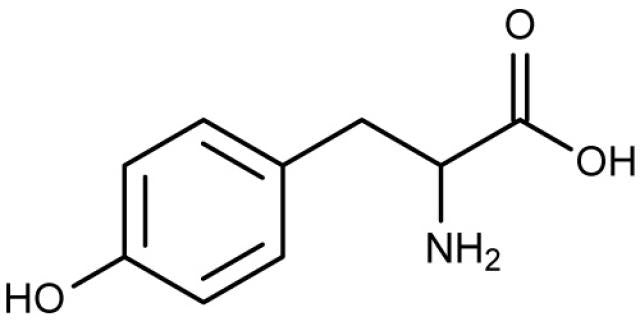
Tyrosine is a nonessential amino acid used to make the catecholamine neurotransmitters dopamine (DA), norepinephrine (NE), and epinephrine (E).

Tyrosine Molecular Structure

Tyrosine Metabolism to Dopamine and Noradrenaline (Norepinephrine)
N-Acetyl Tyrosine (NAT) is the amino acid tyrosine bonded to an acetyl group. The acetyl group must be removed from NAT to form tyrosine and become active in the body.

N-Acetyl Tyrosine Molecular Structure
Tyrosine is not very soluble, meaning it does not dissolve well in water. NAT is much more soluble than Tyrosine; because of this NAT is often used for parenteral nutrition (intravenous feeding) instead of L-tyrosine because solubility is crucial for infusion.
Absorption and Bioavailability
Supplement companies often use NAT instead of L-tyrosine because NAT is much more soluble in water. It is assumed that better solubility means increased bioavailability. Bioavailability is a measure of the amount of a substance that enters the circulation and becomes active in the body. If 100% of a substance reaches circulation and becomes active, then it has 100% bioavailability. In the case of tyrosine vs. NAT, NAT is not more bioavailable than tyrosine.
A single dose of 100mg L-tyrosine can elevate plasma tyrosine levels for up to 7 hours. [1] At a dosage of 100mg/kg, oral tyrosine supplement (powders/pills) has been shown to increase plasma tyrosine concentrations by:
- 130% [2]
- 200% [3]
- 223% [1]
- 276% [4]
***The difference in amount of increase may be due to the form of supplementation (pill, powder, with or without food).
Studies support that oral tyrosine supplementation can substantially increase plasma tyrosine levels. Now let’s look at NAT administration.
When administered intravenously, which results in 100% bioavailability by delivering the substance directly into circulation, one study showed 5000mg NAT to only increase plasma tyrosine levels by 25%. [5] Another study found no increase in plasma tyrosine levels with NAT infusion. [6]
Orally administered tyrosine increases plasma tyrosine levels by 130-276% while NAT administered intravenously only resulted in a 0-25% increase. Clearly tyrosine’s bioavailability is not an issue and NAT is not superior.
So why doesn’t NAT increase tyrosine levels as well as tyrosine? Because the NAT is not being converted to tyrosine. To measure the body’s efficiency in converting NAT to tyrosine, researchers measure the amount of NAT in the urine. The more NAT found in the urine the lower the body’s efficiency in converting NAT to tyrosine. When administered intravenous, one study showed 35% of the administered NAT was excreted as NAT in the urine. [7] Another study found that after four hours, 56% of NAT was excreted unchanged in the urine. [5] A third study showed that 60% of NAT was excreted unchanged in urine. [6] This means that 60% the NAT infused was never converted to tyrosine. Researchers have concluded the usefulness of using NAT to increase tyrosine levels are “not apparent.” [5]
The conversion of NAT to tyrosine is very inefficient making L-tyrosine the superior form of supplemental tyrosine.
Crossing the Blood Brain Barrier (BBB)
One of the supposed benefits of NAT is that it is a better nootropic than L-tyrosine because it can cross the blood brain barrier (BBB) more efficiently… This is false. A study on mice compared the ability of orally administered L-tyrosine, O-phospho-L-tyrosine, L-tyrosine methyl ester, and NAT to increase brain tyrosine levels and found that “N-Acetyl-L-tyrosine was the least effective prodrug tested.” [8] NAT does not cross the BBB better than L-tyrosine and in fact was the least effective form of the four forms of tyrosine tested.
Conclusion
Studies support L-tyrosine supplementation being superior to NAT. Oral L-tyrosine supplementation increases plasma tyrosine levels by 130-276% while intravenous NAT only provides a 0-25% increase. Due to the inefficiency in converting NAT to tyrosine, up to 60% of NAT is excreted in the urine unchanged, meaning it was never converted to tyrosine. Even though NAT is more soluble than L-tyrosine, it is an inferior form of tyrosine supplementation.
So why do companies use N-acetyl tyrosine instead of L-tyrosine? Because it dissolves better in water and they have failed to research the topic adequately.
To ensure optimal cognitive function and performance enhancement, I formulated PR-BREAKER MATERIA with a clinically effective 2-gram dose of L-Tyrosine per serving—far superior to the ineffective N-Acetyl Tyrosine (NAT) used in many other pre-workouts. With MATERIA, there are no shortcuts—just scientifically proven ingredients at the right dosages to fuel your best workouts.
References:
- Glaeser, B.S., et al., Elevation of plasma tyrosine after a single oral dose of L-tyrosine. Life Sci, 1979. 25(3): p. 265-71.
- Smith, M.L., et al., Randomised controlled trial of tyrosine supplementation on neuropsychological performance in phenylketonuria. Arch Dis Child, 1998. 78(2): p. 116-21.
- Pietz, J., et al., Effect of high-dose tyrosine supplementation on brain function in adults with phenylketonuria. J Pediatr, 1995. 127(6): p. 936-43.
- Lykkelund, C., et al., Increased neurotransmitter biosynthesis in phenylketonuria induced by phenylalanine restriction or by supplementation of unrestricted diet with large amounts of tyrosine. Eur J Pediatr, 1988. 148(3): p. 238-45.
- Magnusson, I., et al., N-acetyl-L-tyrosine and N-acetyl-L-cysteine as tyrosine and cysteine precursors during intravenous infusion in humans. Metabolism, 1989. 38(10): p. 957-61.
- Druml, W., et al., Utilization of tyrosine dipeptides and acetyltyrosine in normal and uremic humans. Am J Physiol, 1991. 260(2 Pt 1): p. E280-5.
- Hoffer, L.J., et al., N-acetyl-L-tyrosine as a tyrosine source in adult parenteral nutrition. JPEN J Parenter Enteral Nutr, 2003. 27(6): p. 419-22.
- Topall, G. and H. Laborit, Brain tyrosine increases after treating with prodrugs: comparison with tyrosine. J Pharm Pharmacol, 1989. 41(11): p. 789-91.




Comments
Hello Rene, there are not many studies on oral NAT supplementation unfortunately. Most studies on NAT look at intravenous infusions (and NAT did not raise tyrosine levels significantly in those studies).
Thank you for the info.
Can someone explain to me why the NAT was given intravenously but the L-Tyrosine was given orally?
Why is the method of absorption different?
The bibliography/references noted are from the 1980’s and 90’s (except for one from 2003). Perhaps others are basing their opinions on more recent research?
Edson, absolutely. These studies are more trustworthy than someone’s opinion as they are based on scientific study.
TO MUCH PEOPLE BELIEVE THE OPPOSITE IN ANOTHER SITES. ARE THESE STUDIES TRUSTFUL?
Excellent analysis!! So true! Thank you.
thank you for clearing this topic up for me. I’ve recently begun researching all aminp acid supplementation due to it’s effectiveness fpr treating not only depression and other mood disorders, but fibromyalgia as well, which has been a plague of.mine for some years now at the ripe young age of 31. (joke) Glad you research well and provide the resources you’ve itilized. It’s rate I stumble upon an article that is clear concise and not a “blogger’s” endless rant on a “trending” subject with more opinions than verifiable evidence. I will definitely continue to consult your page on such subjects.
It’s so challenging to find this kind of information on the website – straightforward and substantiated. I can’t thank you enough.
Hey John. We all have access to the same research (the research presented in this article) which clearly states that Tyrosine is superior to N-Acetyl-Tyrosine.